Sensitivity increases with tumour burden and later stage disease
There is a number of tools in the arsenal for the fight against breast cancer—among those, blood tests. How would a disease of the breast be detectable in the blood? This is based on the idea of “tumour markers”. During disease development, cancer cells undergo a number of changes that make them distinguishable from normal healthy cells—for example, they can start over-producing certain proteins, or undergo DNA mutations that drive carcinogenesis. Such “tumour markers” can escape the tumour and enter the blood steam, where they can be detected using a number of molecular diagnostic techniques. Such tests are potentially cheap, relatively non-invasive and could provide a useful supplement for monitoring the disease.
A number of FDA-approved blood tests rely on protein tumour markers. Some of these proteins—such as the carcinoembryonic antigen, CEA—are only found in embryos. Others—the mucin-1 protein (which is detected by both the CA 15-3 and the CA 27-29 blood tests), cytokeratin proteins or the HER-2 protein—have a function in normal adult cells. However, during breast cancer it is believed one or more of these proteins start getting over-produced to abnormal levels, as the disease highjacks them to help tumour growth, invasion and escape from the immune system. Therefore, abnormally elevated levels of these proteins potentially indicates something is amiss.
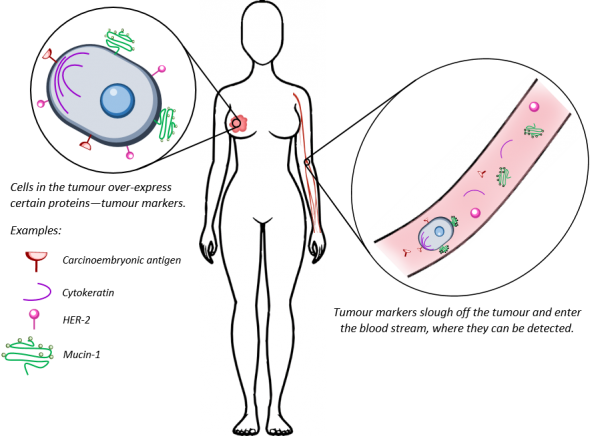
Using blood tests to monitor for such abnormal protein levels is already a clinically acceptable way to detect cancer recurrence or metastasis. The specificity of tumour markers can be very high—93.5% for CA 15-3 and 95% for CEA, when it comes to detecting disease recurrence [1]. CA 15-3 and CA 27-29 have 40-55% sensitivity for cancer recurrence; this improves to 60-70% sensitivity when CEA is considered simultaneously. When it comes to detecting metastatic breast cancer, abnormal levels of CA 15-3, CEA and cytokeratins together produce 90% sensitivity. Another protein that can be used is the HER-2 protein. In the roughly 20% of breast cancers that over-express this protein (HER-2+), the extracellular domain of HER2 is cleaved off and enter the blood stream. Therefore, for patients with HER2+ disease, blood levels of HER-2 are useful for prescribing targeted therapy (such as trastuzumab) and monitoring response to such therapy [2]. However, for all of these tumour marker proteins their levels within circulation (and hence the sensitivity of the blood tests) is directly related to the tumour burden and cancer stage [3]. Hence, while these tests are useful for monitoring disease after it has been diagnosed, the levels of these tumour markers are usually undetectable in early stage disease. Therefore, they are not appropriate for screening purposes and for initial diagnosis of breast cancer.
Entire tumour cells can also be identified within the blood stream. CellSearch, developed by Veridex, is currently the only FDA-approved test that detects such circulating tumour cells (CTCs) [4]. It is currently approved for monitoring metastatic breast cancer; in these patients, detectable levels of CTCs are associated with a 178% decrease in overall survival [5]. However, as with the tests for protein levels, while CellSearch has high specificity, its sensitivity is directly related to tumour burden and thus cannot detect the early presence of breast cancer reliably enough for screening purposes. In metastatic breast disease, the sensitivity of CellSearch ranges from 36 to 70% [6-9]. All of the discussed blood tests should not be used on their own, but in conjunction with breast imaging, physical examination and medical history [2].
Finally, blood tests have a limited role in predicting the risk of breast cancer. Blood is a facile source of DNA and is useful for detecting risk-associated mutations. Carriers of high-risk mutations, such as those situated within the genes BRCA1, BRCA2, PTEN or TP53, have a greatly increased incidence of breast cancer; however, they only constitute approximately 5-10% of all incidences of breast cancer [10]. Therefore, while such testing is a great help in identifying and treating women in this high-risk population, it is not sufficient to identify the vast majority of future breast cancer sufferers whose disease will be sporadic.
A number of new blood tests are in the development pipeline, offering promise of greater sensitivity for breast cancer screening [11, 12], or even prediction of future disease risk [13]. However, such tests are still in the early development stage and existing data on their sensitivity and efficacy is usually based on fairly small sample sizes (fewer than 1 000 subjects). More extensive testing would be needed to firmly establish their clinical utility and reliability. Therefore, while blood tests for breast cancer have current and future clinical utility, they are not yet useful for stand-alone application and cannot be used for disease diagnosis. Clinical modalities such as medical imaging and clinical examination are still very much a requirement in the fight against breast cancer.
1. Molina, R., et al., Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol, 2005. 26(6): p. 281-93.
2. Harris, L., et al., American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol, 2007. 25(33): p. 5287-312.
3. Mirabelli, P. and M. Incoronato, Usefulness of Traditional Serum Biomarkers for Management of Breast Cancer Patients. BioMed Research International, 2013. 2013: p. 9.
4. Janssen Diagnostics. CELLSEARCH® Circulating Tumor Cell Test. 2016 [cited 2016 23 August]; Available from: https://www.cellsearchctc.com/.
5. Matsumoto, A., et al., Biological markers of invasive breast cancer. Japanese Journal of Clinical Oncology, 2015.
6. Muller, V., et al., Prognostic impact of circulating tumor cells assessed with the CellSearch System and AdnaTest Breast in metastatic breast cancer patients: the DETECT study. Breast Cancer Res, 2012. 14(4): p. R118.
7. Van der Auwera, I., et al., Circulating tumour cell detection: a direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT–PCR in patients with metastatic breast cancer. British Journal of Cancer, 2010. 102(2): p. 276-284.
8. De Giorgi, U., et al., Circulating tumor cells and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for outcome prediction in metastatic breast cancer. J Clin Oncol, 2009. 27(20): p. 3303-11.
9. Riethdorf, S., et al., Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Metastatic Breast Cancer: A Validation Study of the CellSearch System. Clinical Cancer Research, 2007. 13(3): p. 920-928.
10. Martin, A.-M. and B.L. Weber, Genetic and Hormonal Risk Factors in Breast Cancer. Journal of the National Cancer Institute, 2000. 92(14): p. 1126-1135.
11. Henderson, M.C., et al., Integration of Serum Protein Biomarker and Tumor Associated Autoantibody Expression Data Increases the Ability of a Blood-Based Proteomic Assay to Identify Breast Cancer. PLoS One, 2016. 11(8): p. e0157692.
12. Mistry, D.A.H., J. Haklani, and P.W. French, Identification of Breast Cancer-Associated Lipids in Scalp Hair. Breast Cancer : Basic and Clinical Research, 2012. 6: p. 113-123.
13. Bro, R., et al., Forecasting individual breast cancer risk using plasma metabolomics and biocontours. Metabolomics, 2015. 11(5): p. 1376-1380.
